Amyloidoses form a large heterogeneous group of diseases such as Alzheimer, Parkinson or kidney amyloidosis. They are characterized by the presence of deposits in a tissue that consist of insoluble aggregated misfolded proteins in the form of fibrils. A typical fiber shape in electron microscopy images and a cross-beta X-ray diffraction pattern identify the deposits.
We acquired an expertise in detecting and analyzing amyloid fibers directly in tissues or organs (Briki 2011). We developed synchrotron X-ray imaging technique to caracterize amyloid molecules inside a tissue.
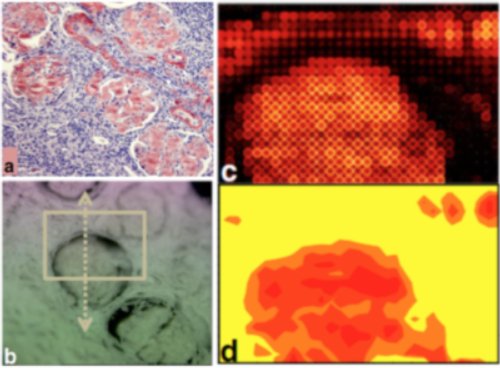
Among issues in the field of amyloid diseases is the understanding of the fibrillation process. Deciphering the fibrillation mechanisms in amyloidosis is a key step to develop anti amyloid agents capable of disrupting the aggregated fibers or simply to prevent their formation.
We used the above approach to understand the fibrillation process in the Aa-chain fibrinogen protein amyloids formed in vivo in people affected with amyloidosis (Garnier 2017). In collaboration with Dr Valleix at Necker Hospital, we combined in silico and in vitro analyses with ex vivo diffraction imaging and we identified a 12-residue amino acid sequence as the one deposited in kidney. Indeed, fine structural analysis of various peptides allowed to demonstrate that 5-residues-peptide VLITL in the abise peptide is the key amyloid prone motif sequence to generate stable amyloid fibers.
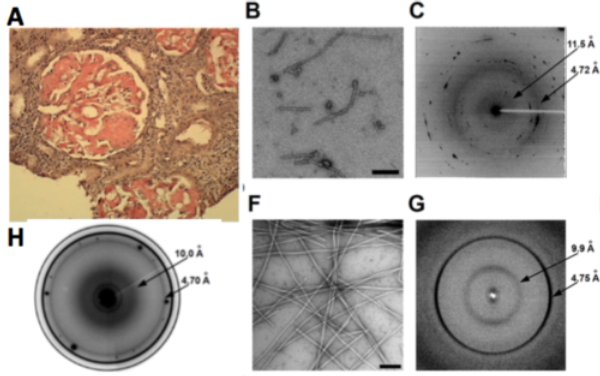
(A) Renal cut stained with congo red. (B) Short fibers from 49-amino-acid deposited peptide in kidney from electron microscopy and corresponding microdiffraction pattern (C) typical of amyloids. (F) Micrograph of fibers from a 12-AA peptide. The resulting diffraction patterns (G) show the same characteristics as the in vivo formed fibers (H). From the shortest peptides (VLITL), common to all the deposited aggregates in kidney, the micrographs show straight fibers and a well-defined powder diffraction pattern with the same periods as the longer peptides characteristic of cross-beta sheet.