Solar cells based on mixed-halide hybrid perovskites show efficiencies that have already surpassed the best polycrystalline silicon-based cells, in just a few years since their discovery 1. However, these advanced perovskite materials still suffer from instability and undergo phase segregation under light illumination, which degrades their optoelectronic properties 2 3 . Recently, an LPS team has reported new insights into the halide ion migration in such a mixed-halide perovskite material when subjected to photo-excitation.
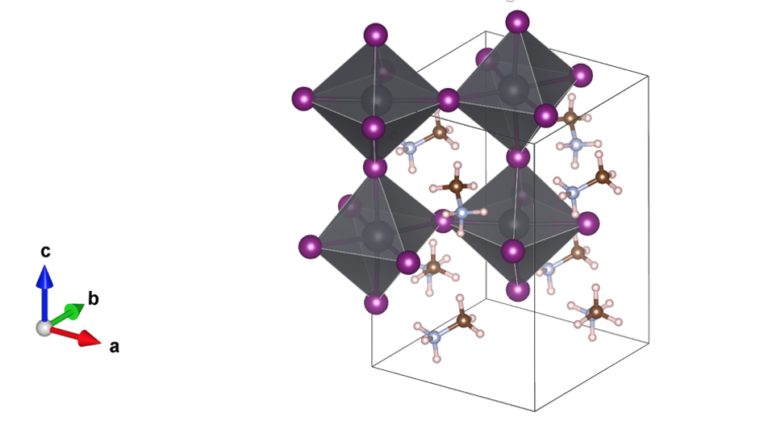
The chemical composition of the triple-cation mixed-halide perovskites (TC-MHP) is (MA0.17 FA0.83)0.95Cs0.05 Pb(I0.83 Br0.17)3 where MA and FA stand for organic molecules (methylammonium and formamidimium), mixed with Cs+ ions within the frame defined by Pb, I and Br ions. This material is an example of a perovskite, with a crystallographic structure which has a generic formula ABX3 (Figure 1), where A is a cation, B is lead in this case, and X is a mixture between bromide and iodide anions. The studied samples are thin polycrystalline films of thickness about 380 nm, which were prepared using spin coating technique on glass substrates.
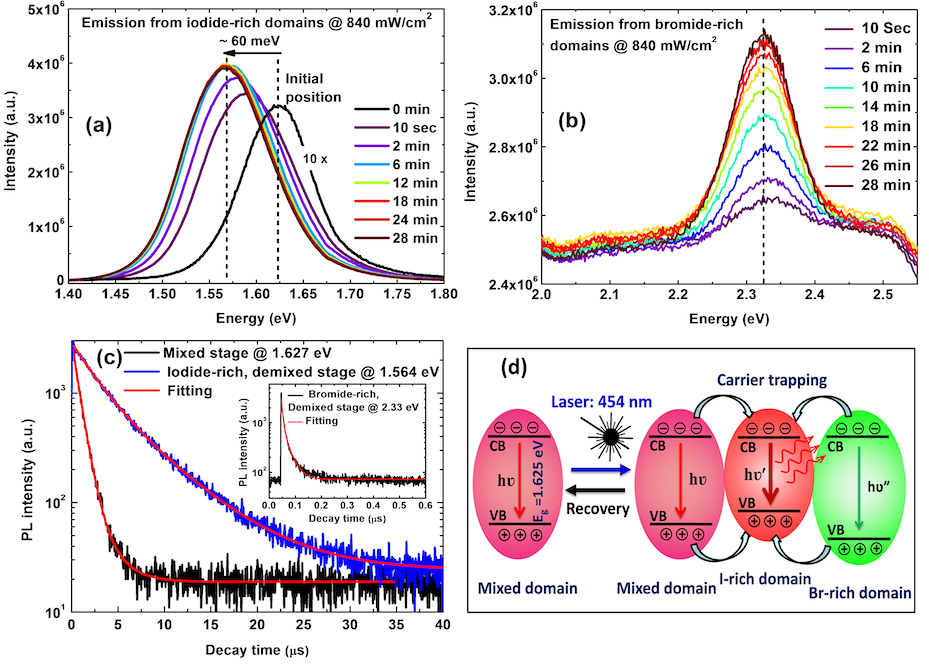
The team studied the thin films using photoluminescence (PL) spectroscopy measurements. PL spectroscopy is an optical method that is concerned with light absorption and light emission from a material, giving insights into the underlying electronic mechanisms responsible for luminescence, i.e. the light emission processes other than thermal radiation. In this study, the PL results reveal the photo-excitation induced halide ions (both Br- and I-) migration in TC-MHP films. This phenomenon is observed through PL spectra red-shift and interpreted as the formation of smaller-bandgap iodide-rich domains in the perovskite film. The band gap is a characteristic of semiconductor materials, which energy value governs the light absorption and emission mechanisms.
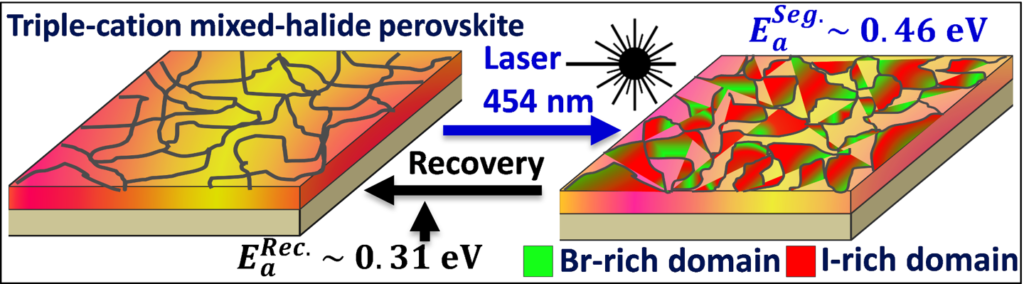
This study reveals that segregated iodide-rich domains efficiently trap the photo-excited-carriers, revealing their dominant role in the origin of unusual long carrier lifetime (average lifetime increases from 0.8 μs to 4 μs under illumination). A long lifetime is usually related to a high conversion efficiency in a solar cell, so this effect would be interesting if the material would be homogeneous. However, in this specific case the long lifetime is a consequence of the carrier recombination in small separated regions (iodide rich), which is detrimental to a good charge collection over a large device. The phase segregation rate is found to follow the excitation power-density as a power law. The threshold excitation power density for phase segregation was estimated using different laser power densities and found to be 2.0 mW/cm2 at room temperature, which is very low as compared for instance to the average solar illumination 100 mW/cm2.
Interestingly, it is found that these photo-induced changes are fully reversible by a thermally activated process when the excitation power is turned off. At room temperature (296 K), a segregated TC-MHP film recovers itself completely in 10 h under darkness. However, it is found that the phase segregation process is frozen out below a temperature T=250 K. A significant difference in activation energies for halide ion migration is observed during phase segregation and recovery process under darkness. The study showed evidence that the activation energy of iodide ion migration is higher during photo-induced phase-segregation process (Ea(Seg.) 0.46 eV) as compared to recovery process (Ea(Rec.) 0.31 eV). Both diffusion mechanisms are supposed to be defect-mediated, as the material is well known for its ability to withstand a high density of intrinsic point defects within its crystallographic structure. These findings will help to understand the key issues of phase segregation in the mixed halide perovskite materials for the development of efficient solar cells and optoelectronic devices.
Reference
Reversible Photo-Induced Phase Segregation and Origin of Long Carrier Lifetime in Mixed-Halide Perovskite Films
S. K. Gautam, M. Kim, D. R. Miquita, J.-E. Bourée, B. Geffroy and O. Plantevin
Advanced Functional Materials (2020) 2002622
doi:10.1002/adfm.202002622