Imogolite nanotubes (INTs) are unique anisometric particles with monodisperse nanometric diameters while the aspect ratio can be controlled by the synthesis conditions. It thus appears as an interesting model system to investigate how aspect ratio and ionic valence influence the colloidal behavior of highly anisometric rods.
In a recent paper published in Journal of Colloid and Interface Science, we demonstrated that divalent (SO42-) or trivalent (PO43-) anions induce aggregation of the nanotubes. The formation of aggregates and their related microstructure does not depend on the nanotube aspect ratio nor the volume fraction. This behavior suggests a heterogeneous aggregation process. By contrast, colloidal dispersions of INTs in NaCl display a rich phase behavior with the occurrence of true liquid-crystal phases (nematic and hexagonal columnar phase) before reaching a glassy-like state (Figure). We also found that the microstructure is affected by the anisometric nature of the particles, allowing to describe the colloidal organization from the dilute state to the arrested phase. The predominance of repulsive interactions is confirmed from calculations of the interaction potential, whose potential decays with decreasing aspect ratio of the nanotubes. This makes imogolite nanotubes attractive as a model system for further experimental and theoretical experiments concerning the colloidal stability of charged 1D nanoparticles and the relation between structure and viscoelastic properties.
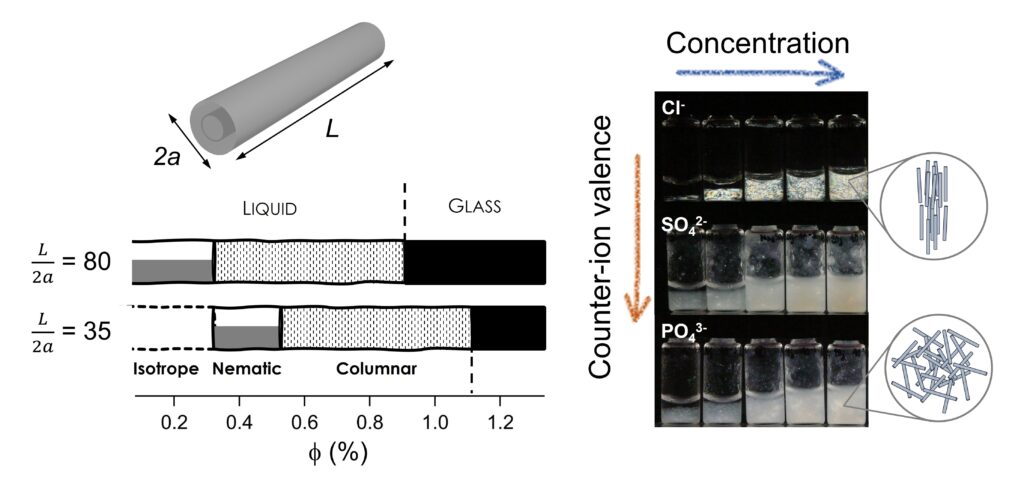
Figure. (left) Phase diagram as a function of INTs volume fraction and for two different aspect ratio. (right) Macroscopic optical observations between crossed polarizers of aqueous dispersions of INTs prepared at different concentration and in different electrolytes.
Reference
Colloidal phase behavior of high aspect ratio clay nanotubes in symmetric and asymmetric electrolytes
C. Hotton, L. Le Roux, C. Goldmann, S. Rouzière, P. Launois, T. Bizien, E. Paineau
Journal of Colloid and Interface Science 664, 857-867 (2024)
DOI: 10.1016/j.jcis.2024.03.046 (open access)
Open archive platforms: HAL; ChemRxiv
