Principal interests
Our research activities are in the field of fundamental physics or physical chemistry, in connection with societal issues such as carbon-free energy. They relate to the structural and dynamical properties of confined molecules, from the original properties of nanoconfined water [1] to energy (H2 production & storage). We also study in depth the nanocontainers themselves [2]. Finally, we consider the use of these nanobricks to realize high performance materials [3],[4].
A key point of our activities is our multi-technique approach combining both X-ray scattering (XRS), inelastic and quasi-elastic neutron scattering, infra-red, Raman & X-ray absorption spectroscopies and X-ray fluorescence with extensive use of synchrotron (SOLEIL, ESRF, Diamond) and neutrons (ILL, ISIS, LLB, FRM2) facilities. Moreover, a significant part of our experiments using X-rays is performed in the laboratory thanks to the design of original set-ups for carrying out in-situ experiments (relative humidity, temperature…). Beyond experimental developments, we also develop specific methodologies to analyze quantitatively XRS diagrams of nano-systems of interest.
Members
Permanent members
- Pascale Launois
(Research director) - Erwan Paineau
(Research associate) - Stéphan Rouzière
(Research Engineer)
Postdocs & PhD
- Yifan Pan
(PhD 2021-2025) - Mohammad Abdelsater
(PhD 2021-2024) - Yassine Naciri
(Postdoc 2021-2023) - Arianna D’Angelo
(PhD 2019-2022) - Joseph Moore
(PhD 2018-2021)
Former team members
- Jian Li
(Postdoc 2020-2021) - Pablo Jimenéz-Calvo
(Postdoc 2019-2020) - Geoffrey Monet
(PhD 2016-2019)
Research thematics
Water nanoconfinement
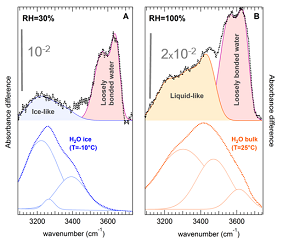
Water molecules, when confined inside nanochannels with diameters comparable to their size or between graphene sheets, exhibit original behaviors with respect to bulk water. For instance, tremendously enhanced water conduction was observed in carbon nanotubes. We studied the spontaneous water-filling of hydrophobic carbon nanotubes, driven by an entropic mechanism, as well as the peculiar hydrogen bond structure of water inside carbon nanotubes, in relation with ultra-low friction of water in these nanotubes (Figure 1).
We also showed that imogolite metal-oxide nanotubes and their functionalized derivatives, synthesized in our team, offer one the rare opportunity to probe the dynamics of confined water in well-defined geometries and for different interactions with the interface. XRS measurements (Figure 2 left) allowed us to determine the lateral dimensions of modified imogolite nanotubes, functionalized by replacing internal hydroxyl with methyl functions, and to prove their hydrophobicity (low density of internal water). Figure 2 (right) illustrates the ability of these nanotubes to capture organic molecules.
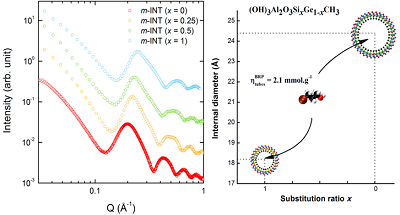
Figure 2. (Left) X-ray scattering diagrams on methyl-modified imogolite nanotubes, de diffusion des rayons X sur des suspensions de nanotubes d’imogolite méthylés, of structural formula (OH)3Al2O3(SixGe1-x)(CH3) and at different substitution ratio x. (Right) Inner diameter of hydrophobic imogolite nanotubes for x = 0 and x = 1. ƞ corresponds to the maximal amount of confined bromopropanol per gram of nanotubes. Adapted from Amara et al., Chem. Mater., (2015), 27, 1488.
Coupling confinement and photocatalysis
Another research path we begun to explore concerns the photo-catalytic activity of INTs. The concept of nanoconfinement-assisted photocatalysis has been studied for several years with the development of 2 and 3 dimensional photo-active nanostructures with permanent polarization. The aim here is to test this concept for one-dimensional confinement, with imogolite nanotubes whose walls are highly polarized, which should allow a good separation of the electron-hole pair with potential applications for the production of H2 or degradation of confined pollutants (Figure 3).
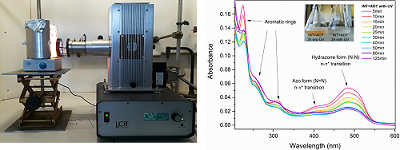
Figure 3. (Left) Solar simulator (Financial support INP, CNRS – EMERGENCE project). (Right) Kinetic monitoring by UV-Vis spectroscopy of the degradation of orange acid 7 by a suspension of imogolite nanotubes.
High-performance fibers with nanotubes
Recent developments in the field of nanomaterials have demonstrated the possibility to produce new fibers with unique properties. Specifically, such systems offer exceptional energy absorption capabilities while maintaining high strength and rigidity. In addition, multifunctional properties including high electrical/thermal conductivity, energy storage, and self-healing can be incorporated. In this context, in collaboration with the team of Prof. M. Shaffer (Imperial College London), we are studying how the properties of imogolite nanotubes can be used to produce new high-performance fibers, particularly with interesting self-healing properties (Figure 4).
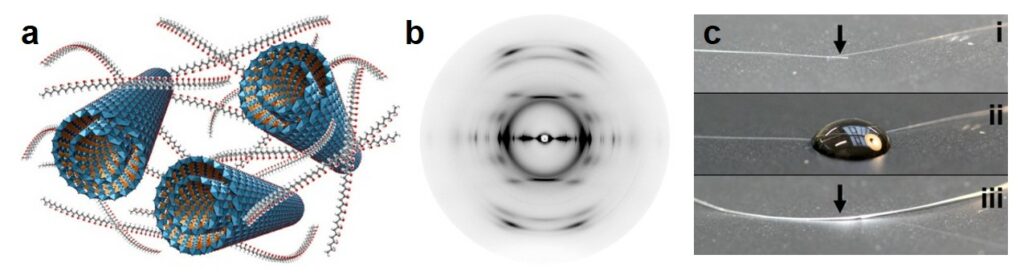
Figure 4. (a) Schematic representation of imogolite nanotubes in a polyvinyl alcohol (PVOH) matrix. (b) Typical X-ray scattering pattern of oriented INT/PVOH fiber. The experiments were carried out on the WAXS-Cu device (MORPHEUS platform). (c) Photographs of the self-healing process by evaporation-induced self-assembly. Adapted from Lee et al., ACS Nano, (2020), 14, 5570.
