Researchers at the Laboratoire de Physique des Solides have studied the self-assembly of liquid-crystal particles inside a droplet when the latter evaporates within an acoustic levitator. The resulting structures formed by drying within the levitator differ remarkably from those obtained on a substrate, making acoustic levitation a powerful technique for shaping materials and rapidly determining phase diagrams.
Evaporation-induced self-assembly techniques have been extensively studied in recent years to organize colloidal particles into ordered materials. Typically, evaporation is performed on a surface leading to an inhomogeneous deposit, known as the “coffee stain” effect. To counter this effect, the scientific community has been working to develop other techniques, such as using a hydrophobic surface, or assembling within a microemulsion or microfluidic channel. However, these approaches face certain limitations, such as the impossibility of controlling droplet size, or the presence of surfactants which can change the surface chemistry of the particles. In this context, acoustic levitation appears to be a promising technique for self-assembling particles in a substrate- and surfactant-free environment, by controlling drop size and producing a 3D assembly.
Researchers at Laboratoire de Physique des Solides set out to compare the self-assembly of liquid-crystal particles obtained by drying on hydrophilic (glass) or hydrophobic (Teflon/FC40) substrates and by acoustic levitation (without substrate). To this end, they used imogolite-type clay nanotubes as a model system, due to their tunable aspect ratio (length/diameter) and rich liquid-crystalline phase behavior. They demonstrated that multiple phase transitions could be achieved by drying in levitated droplets on hydrophobic substrates, while self-assembly was limited on hydrophilic supports. Acoustic levitation offers the advantage over the use of substrates of enabling the kinetics of self-assembly to be studied in-situ, directly on the levitated droplet. Small-angle X-ray scattering experiments carried out in-situ during levitation drying revealed the nature of the crystal-liquid phases (columnar, nematic) and enabled a complete exploration of the phase diagram of these nanotubes, with a limited sample volume (one microliter), in less than an hour, moving from a dilute to a highly concentrated state. The researchers also demonstrated the possibility of modulating the morphology of the materials, from thin films when dried on a glass slide to 3D materials when dried by acoustic levitation or on a hydrophobic substrate. Furthermore, the aspect ratio of the nanotubes is proving to be an essential factor, influencing both the phase transitions and the shape and surface of the resulting materials. Using levitation, the superstructures obtained after drying are less and less spherical, and possess an increasingly rough surface as the aspect ratio increases. This work, demonstrating that acoustic levitation is an emerging technique for shaping colloidal liquid crystal materials and rapidly determining their phase diagram, was recently published in the journal Advanced Materials and Interfaces.
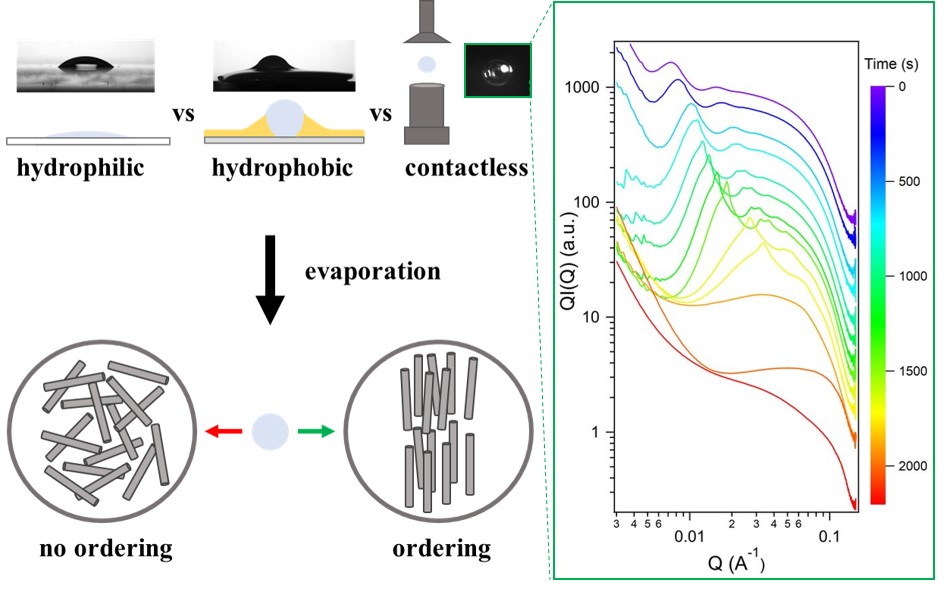
Figure. Comparison of different evaporation-induced self-assembly techniques: on a hydrophilic surface (glass), on a hydrophobic surface (Teflon/FC40) and by acoustic levitation (no substrate). On glass, the nanotubes show no phase transition and remain in isotropic phase when drying begins on a dilute suspension (no ordering). With acoustic levitation, multiple phase transitions are observed during drying (ordering), highlighted by small-angle X-ray scattering (inserted curves).
Reference
Evaporation induced self-assembly of imogolite nanotubes in levitation: exploring phase transitions and material shaping. C. Hotton, T. Bizien, B. Pansu, C. Hamon and E. Paineau
Applied Materials Interfaces, 2024, 2400323
doi: 10.1002/admi.202400323
Contacts
Claire Hotton, Cyrille Hamon & Erwan Paineau
