Unveiling how bacteria modify the surface of solid walls in their close environment at the nanoscale is an important step in nanotechnology and bioengineering as well as in geomicrobiology and fundamental surface chemistry/physics. A multidisciplinary team from the University of Paris-Saclay (LPS and I2BC) has, for the first time, quantitatively linked the stages of corrosion on a metal nanofilm with the actions of bacteria. This work opens new perspectives on the stability and reactivity studies of the first liquid-solid contact nanolayers.
Bacteria have evolved on earth for billions of years and have managed to colonize all ecological niches. Their metabolic diversity is so vast that they play a major role in the biogeochemical cycles of our planet. Understanding the mechanisms by which bacteria interact with the first interfacial layers of the solid matter that surrounds them is an important issue with many perspectives in both fundamental and applied science. Researchers from the LPS opted to work with thin iron films, with a surface area of centimeters and a thickness of nanometers.
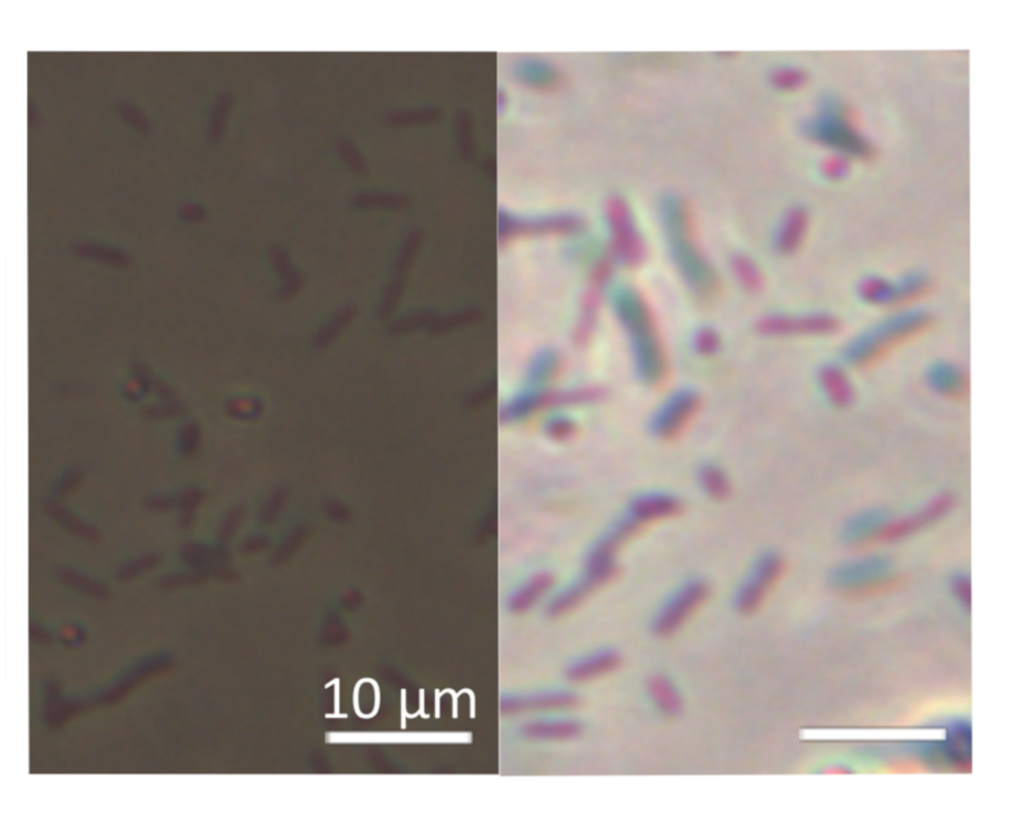
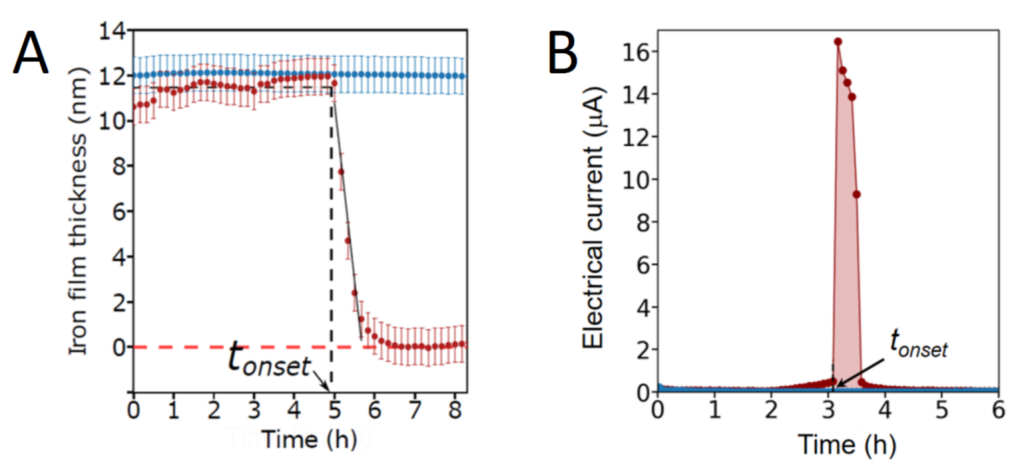
The nanofilm’s opacity being related to its thickness, it is then possible to measure both the nanofilm’s degradation and to localize and quantify the bacteria dynamics in situ and in real time by simple optical means (Figure 1). This experimental work published in the journal ACS Central Science indicates a homogeneous corrosion of iron nanofilms triggered suddenly by bacteria (Figure 2). Experiments reveal in particular rapid motions of Shewanella oneidensis MR1 bacteria during iron dissolution. Mutants of S. oneidensis as well as other bacterial species such as E. coli and L. plantarum are also able to induce corrosion. All the experiments highlight more generally the role of electroactive respiratory proteins and soluble secreted molecules in the modification of the surface properties of nanofilms.
Reference
Biocorrosion on Nanofilms Induces Rapid Bacterial Motions via Iron Dissolution
Marion Lherbette, Christophe Regeard, Christian Marlière, Eric Raspaud
ACS Central Science
DOI : 10.1021/acscentsci.1c01126
Résultat scientifique de l’Institut National de Physique du CNRS
Scoop.it Life Sciences Université Paris-Saclay
Contacts
Eric Raspaud (LPS)
Christophe Regeard (I2BC)