Nanoparticles can assemble in periodic structures, like atoms, called supercrystals. There are many applications for these materials (from catalysis to detection) but they require regular assemblies with as few defects as possible. Despite recent progress, it is still difficult to produce large structures reliably. A team of scientists from the LPS (CNRS/Université Paris-Saclay), in collaboration with two Spanish teams (University of Vigo and CIC Nanogune), have demonstrated the formation of perfectly ordered supercrystals of millimetre size. This work has been published in the journal Advanced Functional Materials.
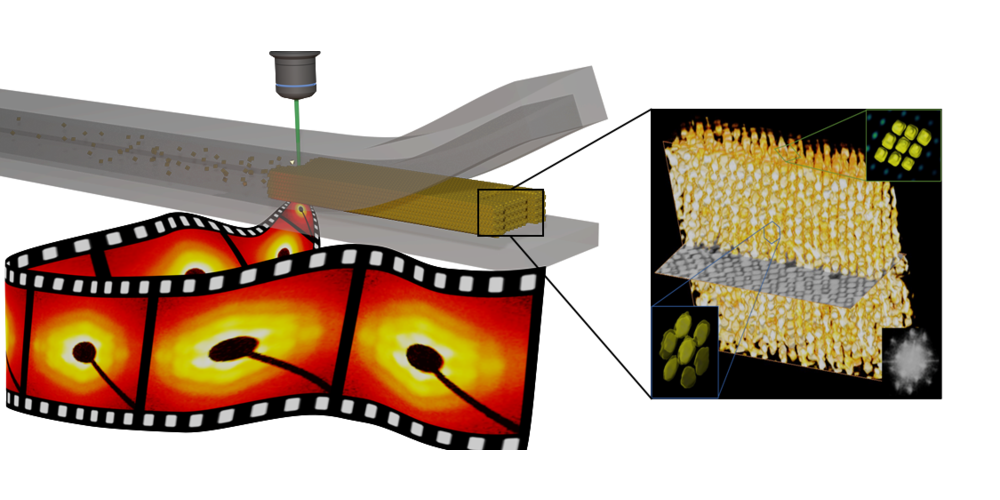
From the variety of shapes that can be synthesised, they chose octahedra. These objects are captivating: while they are quite similar to spheres, they do not form compact structures like the latter. Rather, they self-assemble in three distinct arrays (simple hexagonal, monoclinic or Minkowski array), all of which can sometimes be found in a single sample. This polymorphism probably explains why it is so difficult to obtain regular octahedral stacks. The researchers avoided the problem by confining a solution of particles in a microfluidic channel covered with a thin permeable membrane. During this slow and controlled drying (called pervaporation), they followed the formation of the supercrystal (in time and space) by X-ray scattering. They learned that the process starts with the concentration of nanoparticles in the channel, followed by the nucleation and growth of the supercrystal. The orientation of the supercrystal does not change during growth and it eventually adopts the shape of the channel.
The resulting assembly was studied by electron microscopy: an ion beam cuts the supracrystal and each slice is imaged. The three-dimensional structure can thus be reconstructed and, by combining this information with that obtained by X-ray, the lattice can be identified as monoclinic. Pervaporation therefore stabilises this particular structure at the expense of other possible arrangements and thus allows large supercrystals to be obtained, with potential applications in analytical chemistry.
Reference
Structure and Formation Kinetics of Millimeter-Size Single Domain Supercrystals
Daniel García-Lojo, Evgeny Modin, Sergio Gómez-Graña, Marianne Impéror-Clerc, Andrey Chuvilin, Isabel Pastoriza-Santos, Jorge Pérez-Juste, Doru Constantin & Cyrille Hamon
Adv. Funct. Mater. 2021, 31, 2101869
DOI: 10.1002/adfm.202101869
Contacts
Marianne Impéror-Clerc, Doru Constantin, Cyrille Hamon
